3. Normalisation¶
code
Load data object¶
For the purposes of this demonstration we have generated a smaller data set in which there are only 500 cells per sample. This is so that the code can be run in a reasonable amount of time during the live teaching session. The data were first QC’d and filtered as described in the QC and exploratory analysis session. After this 500 cells were selected at random from each sample.
code
Output
class: SingleCellExperiment
dim: 29346 5500
metadata(1): Samples
assays(1): counts
rownames(29346): ENSG00000243485 ENSG00000238009 ... ENSG00000275063 ENSG00000278817
rowData names(4): ID Symbol Type Chromosome
colnames(5500): 1_CGACTTCGTCCAGTTA-1 1_AGAATAGCATACGCTA-1 ... 8_AGCGTCGAGATGTAAC-1 8_CATGACAAGATAGGAG-1
colData names(10): Sample Barcode ... subsets_Mito_percent total
reducedDimNames(0):
mainExpName: NULL
altExpNames(0):
Why normalise ?¶
code
oneSamTab <- colData(sce) %>%
as.data.frame() %>%
dplyr::filter(SampleName == "PBMMC_1") %>%
dplyr::select(SampleName,Barcode, sum) %>%
mutate(cell_num = 1:n())
p_before_nom <- ggplot(data=oneSamTab, aes(x=cell_num, y=sum)) +
geom_bar(stat = 'identity') +
labs( x= 'Cell Index',
y='Cell UMI counts',
title = "PBMMC_1: Before Normalization" ) +
theme_classic() +
theme(
plot.title = element_text(hjust = 0.5, size=20, color = 'red')
)
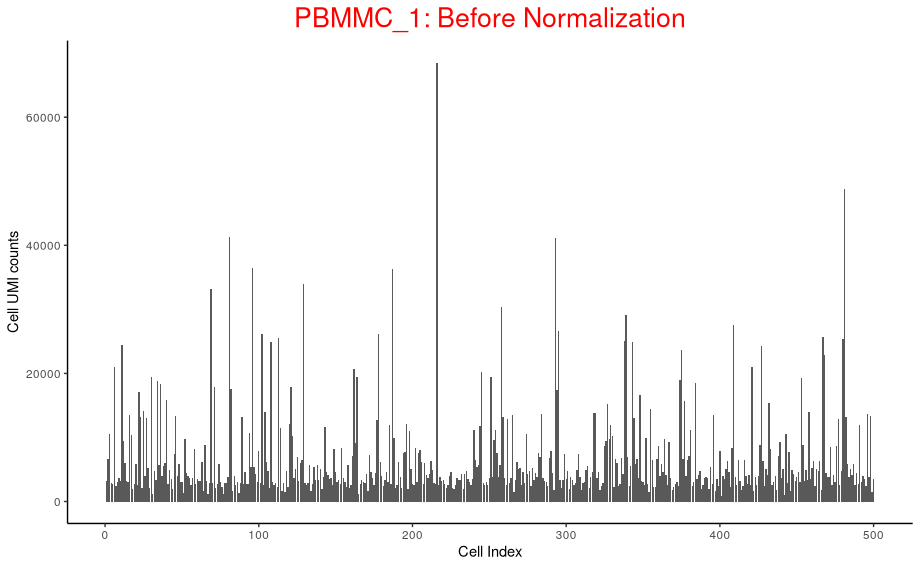
p_before_nom
-
Above plot shows the UMI counts/cell (transcript molecules) for 500 cell from the PBMMC_1 sample
-
UMI counts fluctuate
-
We derive biological insights downstream by comparing cells against each other.
-
But the UMI counts differences makes it harder to compare cells.
-
Why total transcript molecules (UMI counts) detected between cells differ?
-
Biological:
- Cell sub type differences, like size and transcription activity etc.
-
Technical: scRNA data is inherently noisy
- Low mRNA content per cell
- cell-to-cell differences in mRNA capture efficiency
- Variable sequencing depth
- PCR amplification efficiency
-
-
A normalization technique makes the UMI counts distribution uniform, so that each cell has similar counts.
-
Normalization is a critical step that corrects cell-to-cell technical differences.
-
By normalizing, downstream comparisons of relative expression between cells are valid.
Normalization strategies¶
The sparse nature of scRNA data makes normalization difficult, unlike bulk RNAseq data
Broadly two classes:
-
Spike-in methods
- Uses spike-in controls for normalisation
- Not available for droplet based scRNA techniques like 10x.
-
Non-spike-in methods:
- Using available counts data for normalization
- DEseq2
- edgeR-TMM
- Library size normalization
- deconvolution
- sctransform
Typical normalization has two steps:
-
Scaling
- Estimate size or scaling or normalization factor: computation of a cell-specific ‘scaling’ or ‘size’ factor or “normalization factor” that represents the relative bias in that cell and division of all counts for the cell by that factor to remove that bias. Assumption: any cell specific bias will affect genes the same way.
- Scale the data by dividing the count for each gene with the appropriate size factor for that cell
-
Transformation
- log2
- Square root transformation
- Pearson residuals (eg. sctransform)
Scaling methods typically generate normalised counts-per-million (CPM) or transcripts-per-million (TPM) values that address the effect of sequencing depth. These values however typically have a variance that increases with their mean (heteroscedasticity) while most statistical methods assume a stable variance, which does not vary with the mean (homoscedasticity). A widely used ‘variance stabilising transformation’ is the log transformation (often log2). This works well for highly expressed genes (as in bulk RNA-seq) but less so for sparse scRNA-seq data.
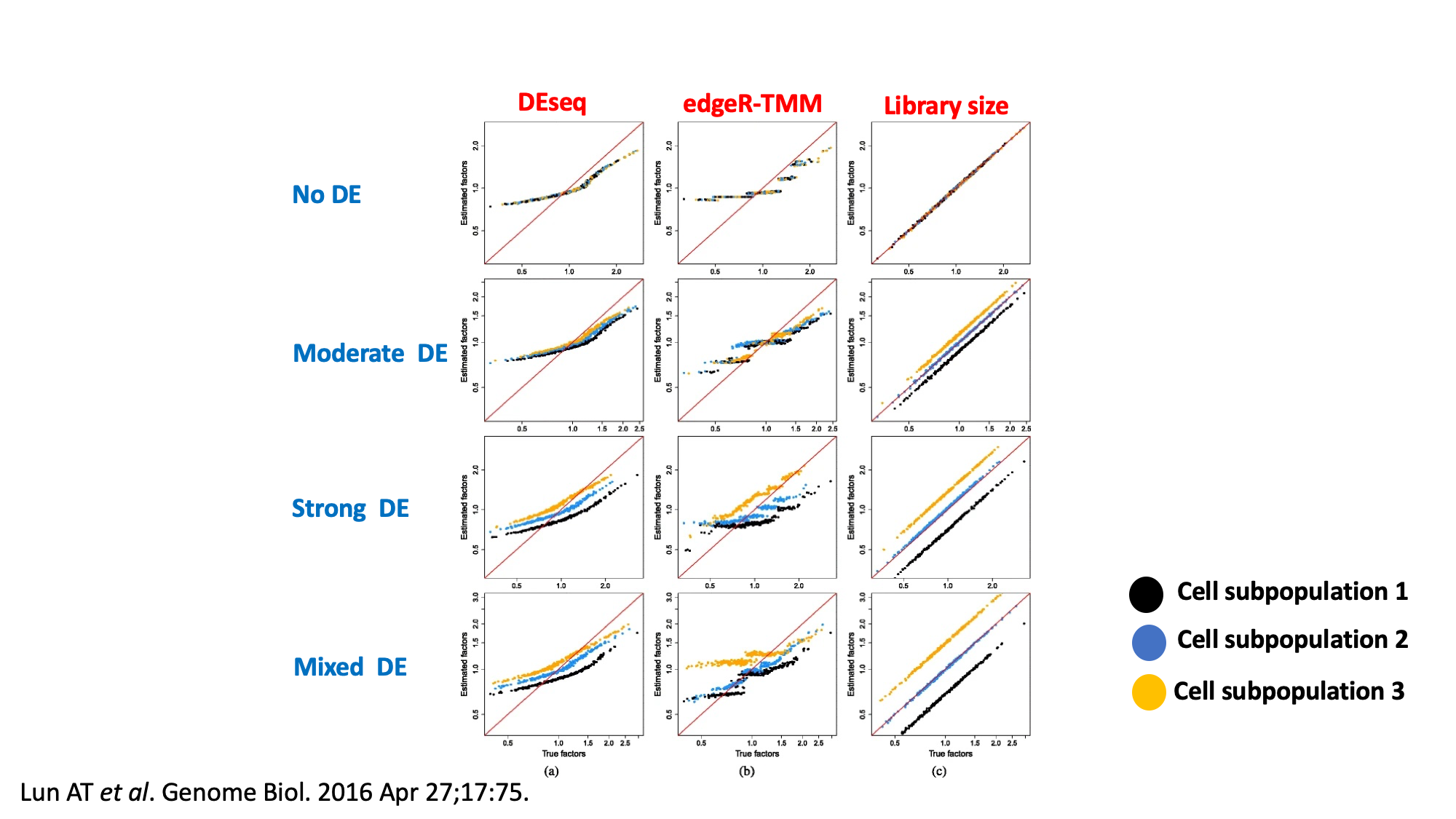
Deconvolution¶
Because single-cell data tend to have a substantial number of low and zero counts, these bulk normalization methods may be problematic for single-cell data.
- Deconvolution aims to normalize expression values based on summed values from pools of cells.
- Since cell summation results in fewer zeros, the ensuing normalization is less susceptible to errors than existing methods.
- The estimated size factors are only relevant to the pools of cells, even though normalization accuracy has improved.
- Each pool’s size factor is deconvolved into its constituent cells’ size factors.
The deconvolution method consists of several key steps:
References: https://genomebiology.biomedcentral.com/articles/10.1186/s13059-016-0947-7
- Defining a pool of cells
- Summing expression values across all cells in the pool
- Normalizing the cell pool against an average reference, using the summed expression values
- Repeating this for many different pools of cells to construct a linear system
- Deconvolving the pool-based size factors to their cell-based counterparts
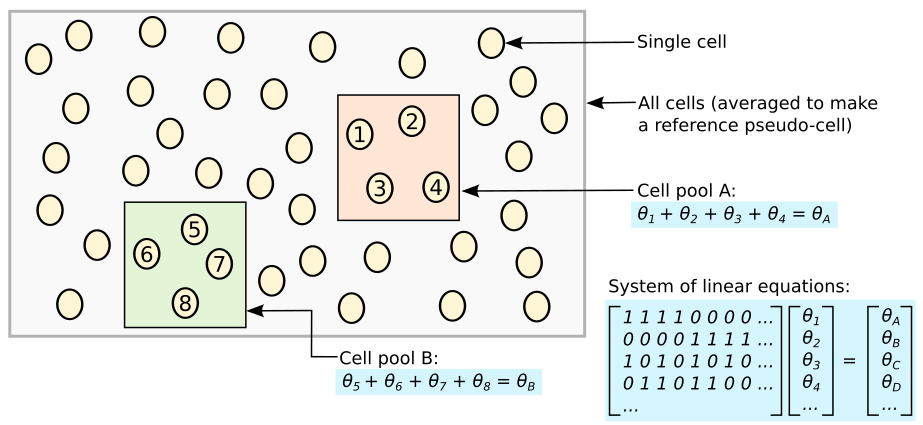
Schematic of the deconvolution method. All cells in the data set are averaged to make a reference pseudo-cell. Expression values for cells in pool A are summed together and normalised against the reference to yield a pool-based size factor θA . This is equal to the sum of the cell-based factors θj for cells j=1–4 and can be used to formulate a linear equation. (For simplicity, the tj term is assumed to be unity here.) Repeating this for multiple pools (e.g., pool B) leads to the construction of a linear system that can be solved to estimate θj for each cell j
In order to avoid pooling cells with radically different transcriptomic profiles, the cells are first clustered based on gene expression. The pools are then formed exclusively with each cluster. Size factors are calculated within each cluster and are then scaled so they are comparable across clusters.
Cluster Cells¶
The table below show the number and size of clusters found:
Compute size factors¶
code
sce <- computePooledFactors(sce,
clusters = clust,
min.mean = 0.1,
BPPARAM = bpp)
deconv.sf <- sizeFactors(sce)
summary(deconv.sf)
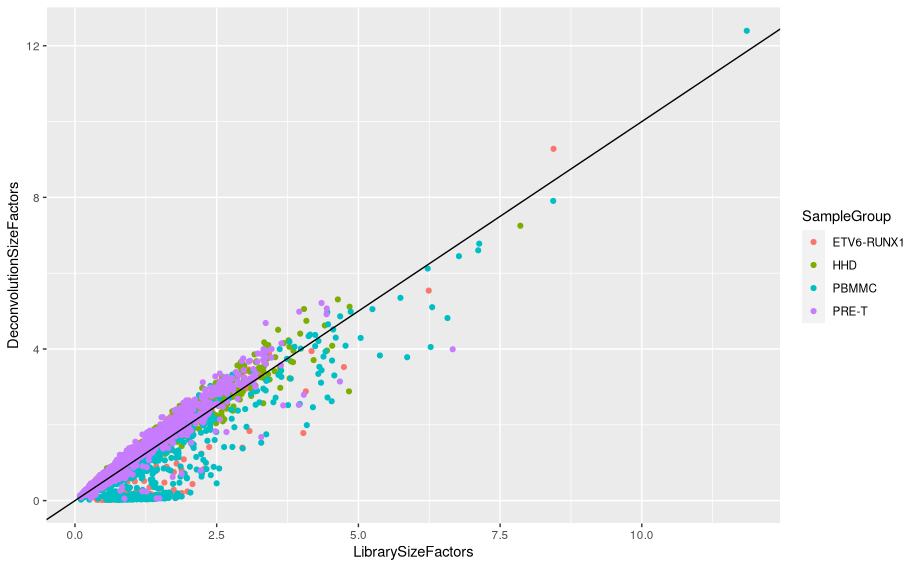
Plot deconvolution size factors against library size factors:
lib.sf <- librarySizeFactors(sce)
data.frame(LibrarySizeFactors = lib.sf,
DeconvolutionSizeFactors = deconv.sf,
SampleGroup = sce$SampleGroup) %>%
ggplot(aes(x=LibrarySizeFactors, y=DeconvolutionSizeFactors)) +
geom_point(aes(col=SampleGroup)) +
geom_abline(slope = 1, intercept = 0)
Apply size factors¶
For each cell, raw counts for genes are divided by the size factor for that cell and log-transformed so downstream analyses focus on genes with strong relative differences. We use scater::logNormCounts().
Explore the effect of normalisation¶
Normalised counts are much less variable across cells than raw counts
code
norm_counts <- logNormCounts(sce,transform='none' ) %>%
assay('normcounts') %>%
as.matrix() %>%
colSums()
norm_counts <- tibble(Barcode=names(norm_counts),
normCounts = log2(norm_counts)
)
norm_counts <- inner_join(norm_counts, oneSamTab, by='Barcode')
p_after_norm <- ggplot(data=norm_counts, aes(x=cell_num, y=normCounts)) +
geom_bar(stat = 'identity') +
labs( x= 'Cell Index',
y='Normalized Cell UMI counts',
title = "PBMMC_1:After Normalization" ) +
theme_classic() +
theme(
plot.title = element_text(hjust = 0.5, size=20, color = 'red')
)
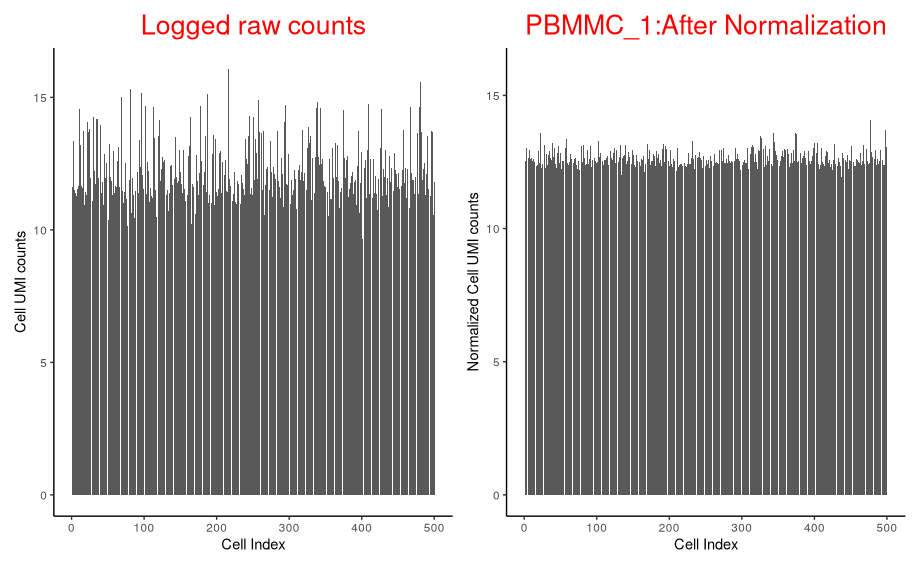
p_before_nom + p_after_norm
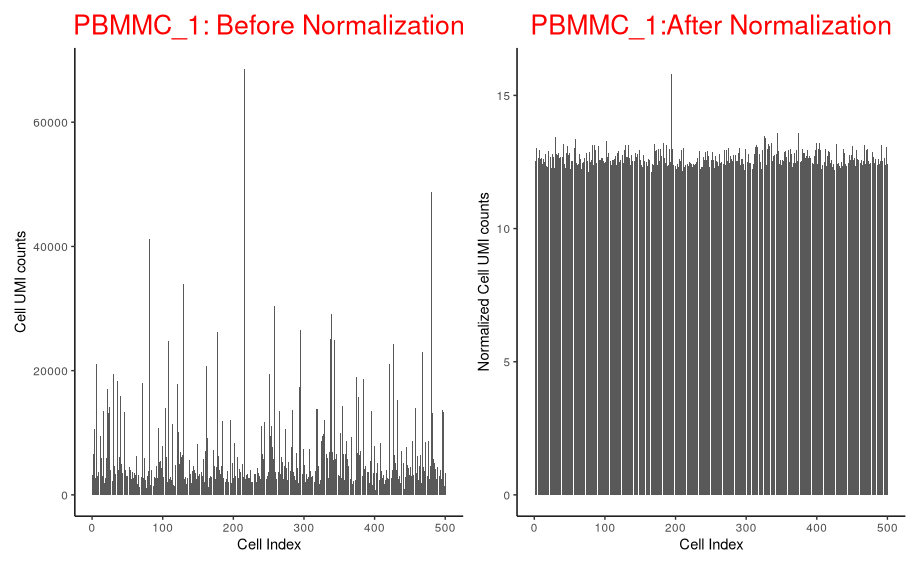
Let’s separate out the scaling normalisation from the log transformation
What do the un-normalised data look like if we log them?
p_before_norm_log <- ggplot(data=oneSamTab, aes(x=cell_num, y=log2(sum))) +
geom_bar(stat = 'identity') +
labs( x= 'Cell Index',
y='Cell UMI counts',
title = "Logged raw counts" ) +
theme_classic() +
theme(
plot.title = element_text(hjust = 0.5, size=20, color = 'red')
)
p_before_norm_log + p_after_norm
Simply logging the sum of counts per cell reduces the variation a lot, but the scaling is required to do the job properly.
The log transformation is meant to reduce the correlation between mean and variance for genes - has this worked?
We can look at the relationship between the mean gene expression and variance for raw UMI counts, scaled counts and scaled, logged counts.
For raw counts:
# mean and variance for raw counts
mean <- rowMeans(assay(sce, "counts"))
var <- rowVars(assay(sce, "counts"))

There is a strong linear relationship between mean and variance of UMI counts across genes.
For scaled counts:
# Mean and variance for scaled counts
mean_scaled <- logNormCounts(sce,transform='none' ) %>%
assay('normcounts') %>%
rowMeans()
var_scaled <- logNormCounts(sce,transform='none' ) %>%
assay('normcounts') %>%
rowVars()
plot(log(mean_scaled), log(var_scaled), main = 'scaled data')
abline(a=1, b=1, col="red")
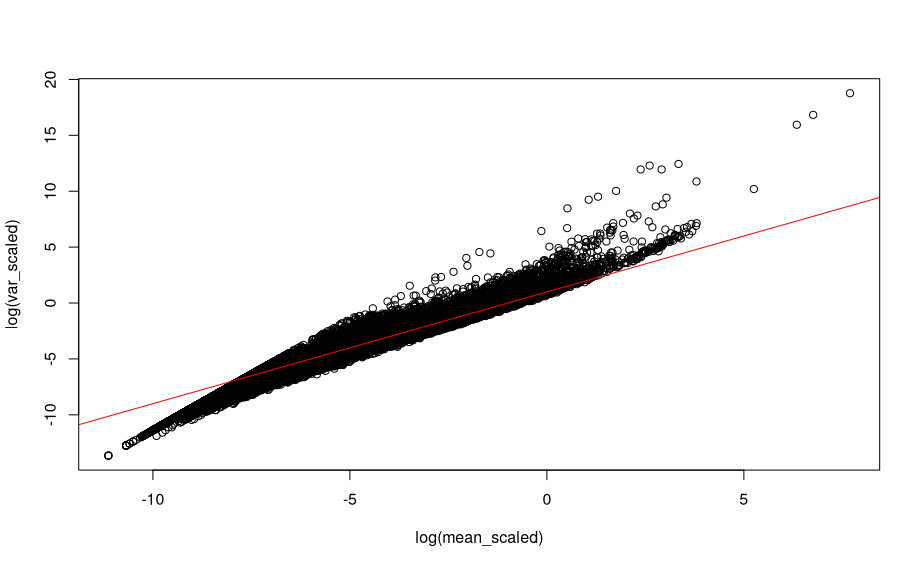
The relationship is still there after scaling the counts.
For scaled, log transformed counts:
# Mean and variance for scaled, log transformed counts
mean_norm <- rowMeans(assay(sce, "logcounts"))
var_norm <- rowVars(assay(sce, "logcounts"))
plot(mean_norm, var_norm, main = 'scaled, log transformed)
abline(a=1, b=1, col="red")
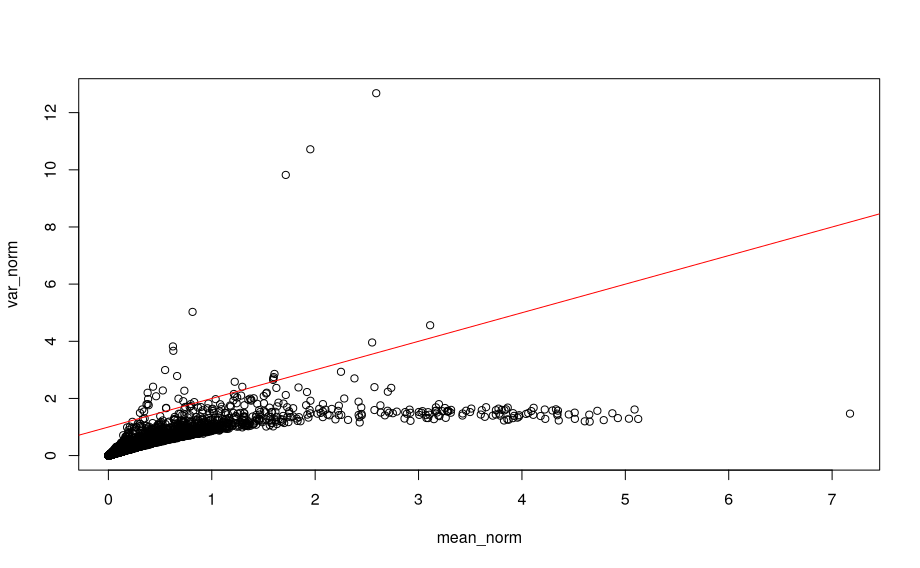
We see that the log transformation removes a large part of the relationship between mean and variance for gene expression values.
Save the normalised object¶
Make sure to create the results directory first.
Exercise
Apply the deconvolution normalisation on a single sample: ETV6-RUNX1_1.
You will first load the a single cell experiment object containing the entire Caron data set. First it is necessary to select only cells that came from the sample ‘ETV6-RUNX1_1’. You can then apply the normalization by deconvolution by clustering the cells, computing size factors and using these to log-normalize the counts.